Understand the abiotic production of redox disequilibrium pairs
The core of this WPl is a series of novel experiments that will likely convert a redox-neutral phosphorus-containing species into phosphine and some oxidizing species. The unknown is whether this abiotic source of redox disequilibrium is efficient enough to result in detectable abundances of products, and if so, under what conditions.
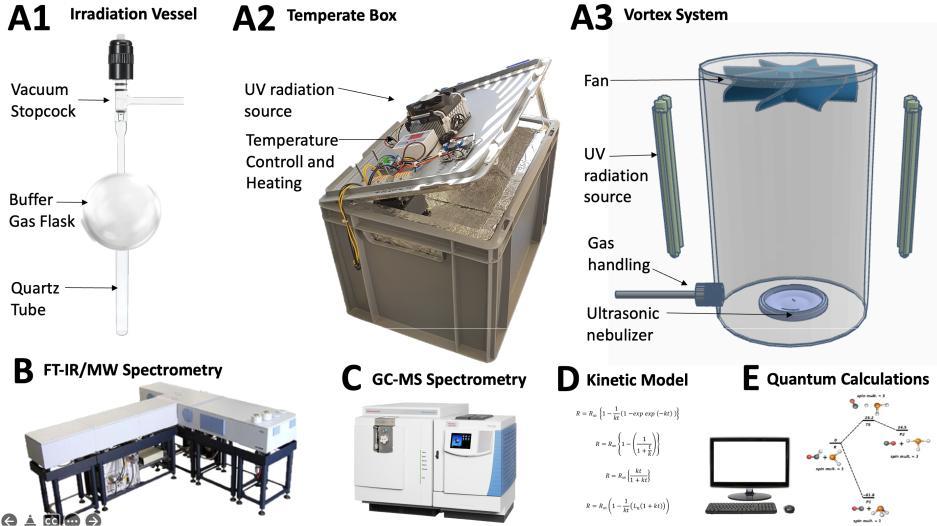
In general, UV reduction of an oxide in the presence of a source of hydrogen leads to the creation of its counterpart hydride, which is reduced, and another species, typically a mineral, that is oxidized, a redox disequilibrium pair (RDP). We have successfully applied this principle to produce CH4 and perchlorates at Mars, and we intend to test this principle for a Venus-analogue environment to see if our experiments produce the RDPs observed in the Venusian clouds. Showing this in the lab would demonstrate the first example of an abiotic source of redox disequilibrium in an atmosphere requiring a reassessment of how biosignatures, even pairs of biosignatures, should be interpreted.
The efficiency of heterogenous photochemical synthesis of other hydrides (PH3, NH3, and H2S) like the origin of CH4 over acidic surfaces must be explored, and we have to answer the fundamental question: Is this likely or not? Particularly for PH3, we already predicted the feasibility of this process through quantum chemical calculations. We will perform similar calculations to explain the observed chemistry in the Venusian atmosphere and to inform the experimental design. The question above is one of the central topics of this project and the answer can be both positive, partly positive, or fully negative.